In a groundbreaking study, TIM-3 Alzheimer’s treatment has emerged as a promising avenue for addressing the cognitive decline associated with Alzheimer’s disease. Researchers have highlighted the TIM-3 molecule’s potential in regulating the hyperactive immune response in the brain, which plays a critical role in plaque accumulation. By inhibiting this molecule, scientists have demonstrated remarkable cognitive improvement in laboratory mice, a significant leap toward new therapies aimed at restoring memory. This innovative immune system therapy draws on strategies previously successful in cancer treatment, suggesting a new interdisciplinary approach to tackling neurodegenerative diseases. As the research progresses, TIM-3 may not only illuminate pathways for Alzheimer’s intervention but also reshape our understanding of neuroinflammation and its ramifications.
Recent advancements in the treatment of neurodegenerative disorders have brought renewed attention to TIM-3 Alzheimer’s therapy, which leverages mechanisms initially targeting cancer. By focusing on the TIM-3 checkpoint molecule, researchers are exploring novel ways to enhance immune function within the brain, facilitating the clearance of toxic amyloid plaques inherent to Alzheimer’s pathology. This therapy embodies a strategic union between immune modulation and cognitive restoration, potentially transforming conventional approaches to Alzheimer’s disease management. Through the modulation of microglial activity, scientists aim to reverse cognitive impairment, marking a shift in how we perceive and combat age-related memory loss. Ultimately, the integration of immune system therapy into Alzheimer’s treatment could usher in a new era of hope for millions affected by this debilitating condition.
Understanding TIM-3’s Role in Alzheimer’s Treatment
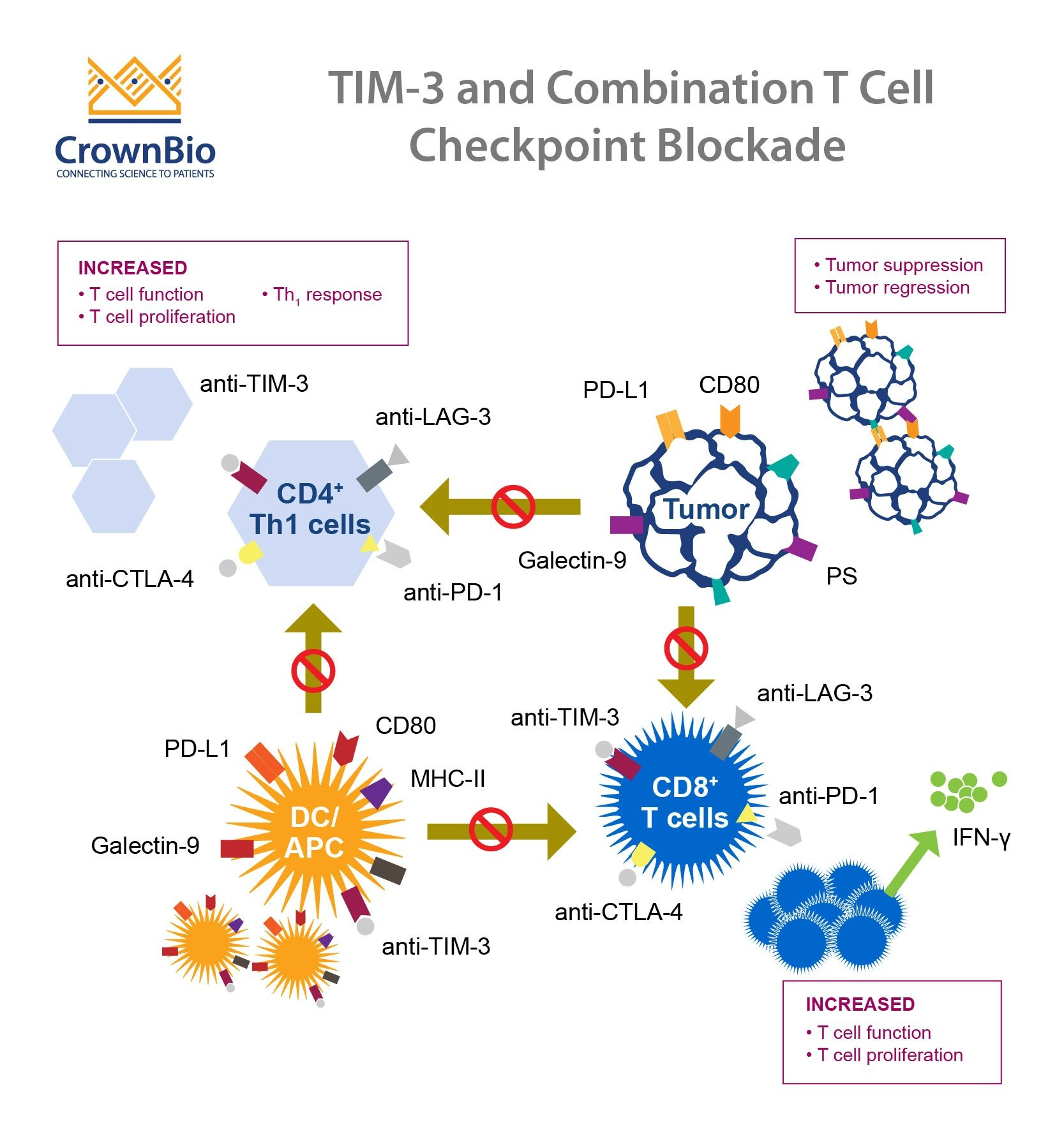
Recent research has identified the TIM-3 molecule as a significant factor in the development and progression of Alzheimer’s disease (AD). This immune checkpoint molecule, traditionally known for its role in cancer treatment, has been shown to inhibit the activity of microglia. These brain immune cells are crucial for clearing amyloid plaques, which accumulate and are associated with the cognitive decline seen in Alzheimer’s patients. In studies, researchers genetically altered mice by deleting the gene responsible for TIM-3, leading to improved memory function and cognitive abilities as the microglial cells could now effectively attack and digest plaques.
The findings provide hope that therapies targeting TIM-3 could translate to Alzheimer’s treatment in humans. By neutralizing TIM-3’s inhibitory effects, it may be possible to reactivate the brain’s immune response, allowing for better clearance of toxic plaques. This approach not only highlights the potential of re-purposing cancer immunotherapy strategies into neurodegenerative conditions but also underscores the importance of understanding the immune system’s role in Alzheimer’s disease.
The Intersection of Cancer Therapy and Alzheimer’s Research
The parallels between strategies used in cancer therapy and potential Alzheimer’s treatments have begun to unravel a new frontier in medical research. Checkpoint inhibitors have revolutionized cancer treatment by preventing tumor cells from evading the immune system. Now, researchers are investigating how similar mechanisms can be employed to combat Alzheimer’s disease by utilizing immune modulation. The TIM-3 molecule, primarily recognized in oncoimmunology, plays a crucial role in regulating microglial functions and is being explored as a target for therapy aimed at plaque removal in Alzheimer’s.
Innovations in this arena can potentially lead to breakthroughs that address both cognitive impairment and the underlying pathological features of Alzheimer’s disease. By understanding how to manipulate these signaling pathways, scientists hope to enhance microglial activation and plaque clearance. This could ultimately pave the way for novel treatments that not only address symptoms but also the fundamental biological disturbances at the root of Alzheimer’s.
Mechanisms of Microglial Activation in Alzheimer’s
Microglia, the brain’s resident immune cells, play a critical role in maintaining neurological health by regulating synapses and clearing harmful substances, such as amyloid beta plaques that accumulate in Alzheimer’s patients. The role of TIM-3 in suppressing microglial activity is particularly noteworthy; its overexpression hinders the ability of microglia to perform their defensive functions. As Alzheimer’s disease progresses, the buildup of plaques suppresses memory and cognitive function, showcasing the need to restore microglial functionality for effective treatment.
Research indicates that by targeting TIM-3, scientists may enable microglia to resume their role in plaque clearance, which could significantly slow disease progression. By understanding the mechanisms governing microglial activation and the inhibitory role of TIM-3, researchers are developing therapeutic strategies that may lead to cognitive improvements and a better quality of life for Alzheimer’s patients.
The Genetic Implications of TIM-3 in Alzheimer’s Disease
Genetic research has shown that specific polymorphisms in the TIM-3 gene are associated with an increased risk of developing late-onset Alzheimer’s disease. This genetic link suggests that TIM-3 not only serves as a physical barrier to microglial action but could also provide insights into which patients might benefit most from TIM-3-targeted therapies. Understanding how genetics influences the expression of TIM-3 in the brain’s immune cells may be crucial for identifying at-risk populations and tailoring preventive or therapeutic strategies accordingly.
Studies are beginning to explore the relationship between TIM-3 variants and cognitive decline, thereby providing further evidence of the gene’s role in Alzheimer’s pathology. In light of these findings, there is potential for developing precision medicine approaches, whereby patients with specific TIM-3 related genetic markers could receive targeted immune-based therapies designed to rectify the defects caused by the overactive inhibitory functions of TIM-3.
Potential Future Therapies Targeting TIM-3
The therapeutic potential of anti-TIM-3 antibodies is gaining traction as researchers explore their application in Alzheimer’s treatment. These targeted therapies aim to block the TIM-3 inhibitory signal, thus permitting microglia to effectively clear amyloid-beta plaques in the brain. If successful, such interventions could lead to significant cognitive improvements in patients suffering from Alzheimer’s disease. Current studies are testing the efficacy of these antibodies in mouse models, which serve as a crucial step towards eventual clinical trials.
Legal and therapeutic frameworks surrounding the use of immune checkpoint inhibitors have expanded alongside their increasing application in oncology. The transition of TIM-3-based therapies from cancer applications to Alzheimer’s treatment reflects an innovative approach to common immunological pathways. Not only could this shift enhance our understanding of Alzheimer’s disease, but it may also redefine treatment protocols, shifting focus from symptomatic care to addressing disease mechanisms at a cellular level.
Measuring Cognitive Improvement in Mice
To assess the effectiveness of TIM-3 inhibition, researchers have developed various behavioral tests measuring cognitive function in mouse models. These tests often involve maze navigation, memory retention assessments, and evaluations of anxiety-like behaviors in stressful environments. Such methods allow scientists to quantify cognitive improvement following interventions that enhance microglial activity and plaque clearance. Observations from these tests can provide essential insights into the neurobiological changes associated with TIM-3 deletions.
Cognitive testing in mouse models serves as a vital precursor to human trials, helping to validate the potential efficacy of TIM-3-targeted therapies. Should these interventions demonstrate marked cognitive improvements in mice, it could catalyze further research and eventual clinical trials aiming to translate these findings into effective treatments for Alzheimer’s patients.
Broader Implications of Immune System Therapies
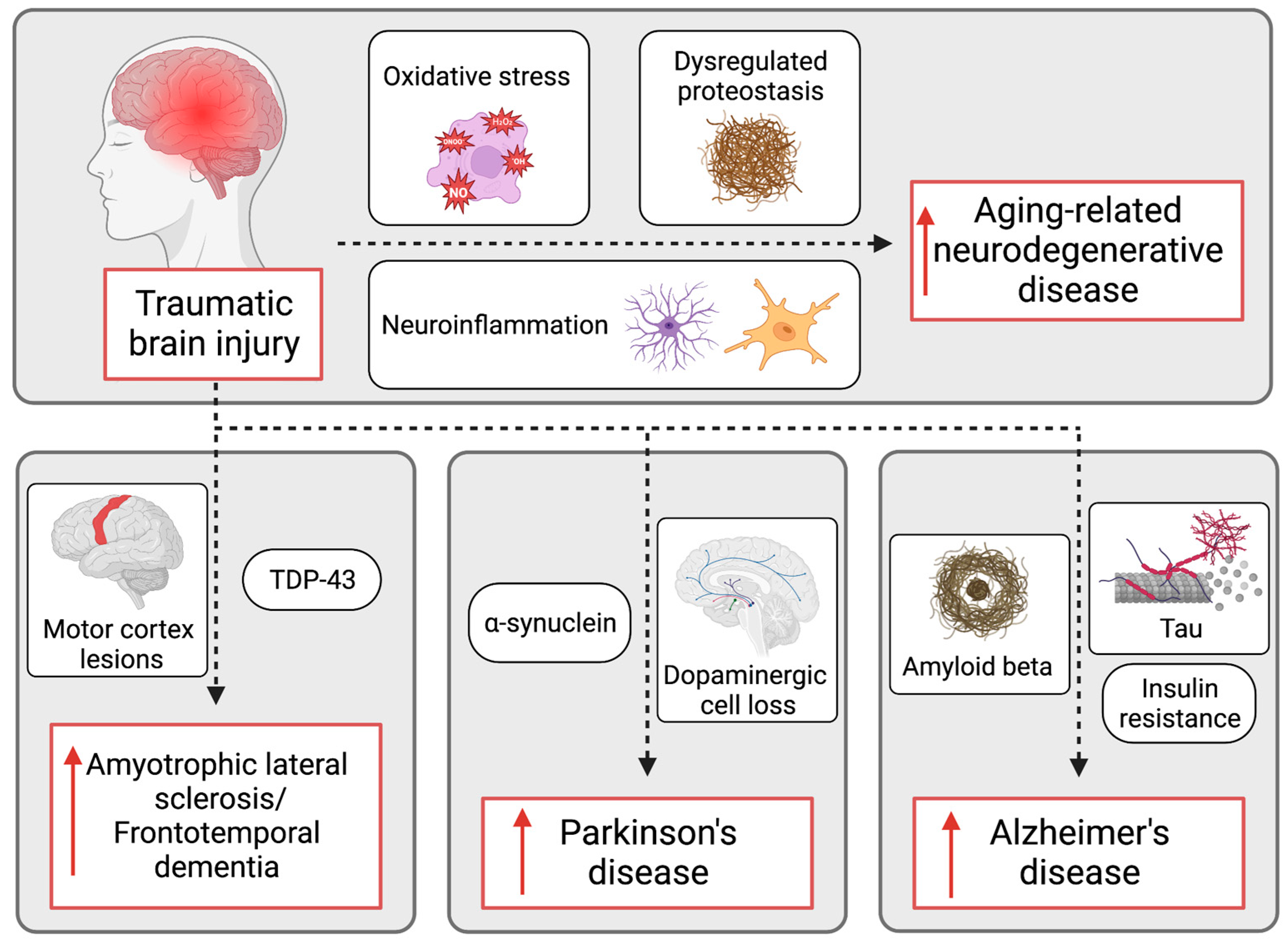
The emerging field of immunotherapy suggests that harnessing the immune system’s potential may offer new avenues in treating chronic neurodegenerative diseases like Alzheimer’s. With the success of TIM-3 inhibition in mouse studies, there is a strong rationale for the continued exploration of immune checkpoint molecules to improve cognitive function and alter disease progression. This approach could unify treatment protocols across various conditions affected by immune dysregulation, such as Alzheimer’s and Parkinson’s diseases.
Understanding the broader implications of these findings can influence not just therapeutic strategies but also research funding and public health policies regarding neurodegenerative diseases. Emphasizing the connection between cancer treatment methodologies and Alzheimer’s research may facilitate interdisciplinary collaboration and accelerate the translation of research into clinical practice, ultimately benefiting patients.
Implementing Clinical Trials for Alzheimer’s Therapies
With the promising results from animal studies targeting the TIM-3 pathway, moving towards human clinical trials becomes the next logical step. Researchers aiming to recruit diverse participant populations will need to address both the safety and efficacy of anti-TIM-3 therapies in real-world settings. This involves careful selection of dosages and monitoring patients for any adverse effects while establishing guidelines for duration of treatment and metrics for assessing cognitive improvement.
The potential for human trials to validate the effects of TIM-3 inhibition could mark a significant shift in Alzheimer’s treatment approaches. If successful, this strategy not only represents a new therapeutic option but could pave the way for further research into similar immune-based therapies, reinforcing the importance of the immune system in maintaining cognitive health and fighting neurodegeneration.
Insights from a Decade of Alzheimer’s Research
Over the last decade, research on Alzheimer’s disease has undergone significant evolution, revealing much about its complex pathophysiological mechanisms. The discovery of molecules like TIM-3 has opened doors to innovative strategies that leverage the immune response to address the underlying causes of cognitive decline. These advancements highlight the shift towards a deeper understanding of the immunological factors involved in neurodegeneration.
As we continue to explore the interplay between the immune system and neurodegenerative diseases, the lessons learned from Alzheimer’s research may yield insights applicable to other disorders. The successful transition of cancer treatment methodologies into Alzheimer’s therapy exemplifies how interdisciplinary research can catalyze scientific breakthroughs and enhance patient care.
Frequently Asked Questions
What is TIM-3 and how does it relate to Alzheimer’s treatment?
TIM-3, or T-cell immunoglobulin and mucin-domain containing protein 3, is an immune checkpoint molecule found to play a significant role in Alzheimer’s treatment. In Alzheimer’s disease, TIM-3 inhibits microglial cells from clearing amyloid plaques in the brain, which are toxic proteins associated with cognitive decline. By targeting TIM-3, researchers are exploring new therapies that could enhance the immune response to restore cognitive function.
How does TIM-3 blockade improve cognitive functions in Alzheimer’s disease models?
Blocking TIM-3 allows microglial cells to become active again, enabling them to clear amyloid plaques effectively. Studies have demonstrated that in mouse models of Alzheimer’s, deleting the TIM-3 protein leads to a significant reduction in plaque levels, resulting in improved memory and cognitive performance in tasks like maze navigation.
Is TIM-3 involved in both cancer and Alzheimer’s disease therapies?
Yes, TIM-3 is an immune checkpoint molecule originally studied for its role in cancer treatment, where it prevents T-cells from attacking tumors. The same inhibitory mechanism is detrimental in Alzheimer’s disease, as it prevents microglia from clearing harmful plaques. This dual role makes TIM-3 a promising target for therapies aimed at both conditions.
What are the potential therapies targeting TIM-3 for Alzheimer’s disease?
Potential therapies may involve the use of anti-TIM-3 antibodies or small molecules that inhibit the function of TIM-3. These therapies aim to reactivate the immune response, enabling microglia to clear amyloid plaques and improve cognitive function in individuals with Alzheimer’s disease.
How significant is the role of TIM-3 in late-onset Alzheimer’s disease?
TIM-3 is significantly implicated in late-onset Alzheimer’s disease, which accounts for approximately 90-95% of cases. Genetic studies have identified polymorphisms in the TIM-3 gene as a risk factor for Alzheimer’s, suggesting that targeting this molecule could potentially alter disease progression.
What evidence supports TIM-3 as a target for Alzheimer’s treatment?
Research in animal models has shown that genetic deletion of TIM-3 improves plaque clearance by microglia and enhances cognition. The findings indicate that TIM-3’s role in inhibiting microglial activity is a crucial mechanism underlying the accumulation of amyloid plaques in Alzheimer’s disease.
Are there ongoing clinical trials for TIM-3 therapies in Alzheimer’s disease?
Yes, researchers are actively investigating the use of human anti-TIM-3 antibodies in clinical trials to halt plaque development in Alzheimer’s disease. Recent studies have laid the groundwork for testing candidate therapies in mouse models with humanized TIM-3 genes.
What challenges do TIM-3 treatments for Alzheimer’s disease face?
One of the main challenges is ensuring the effective delivery of anti-TIM-3 antibodies to the brain, as previous treatments targeting amyloid beta have faced difficulties due to vascular complications. However, TIM-3’s selective expression in microglia may provide a more targeted therapeutic approach.
| Key Points |
|---|
| Harvard research indicates TIM-3 may offer a new treatment strategy for Alzheimer’s disease by enhancing microglial function to clear amyloid plaques. |
| TIM-3 is an immune checkpoint molecule that inhibits microglia, preventing them from attacking harmful plaques. |
| 90-95% of Alzheimer’s cases are late-onset, with TIM-3 being a genetic risk factor identified in studies. |
| Deletion of TIM-3 in mice improved plaque clearance and restored cognitive function, indicating potential therapeutic pathways. |
| The approach may repurpose existing anti-TIM-3 antibodies for human trials, promising a new avenue for Alzheimer’s treatment. |
Summary
The TIM-3 Alzheimer’s treatment represents a groundbreaking approach that leverages an existing immune system strategy to combat Alzheimer’s disease. By targeting the TIM-3 molecule, researchers have shown promising results in enhancing memory and cognitive function in animal models. This innovative method not only has the potential to clear harmful amyloid plaques but could also redefine therapeutic strategies for late-onset Alzheimer’s, a condition affecting the majority of Alzheimer’s patients. As research continues, TIM-3 may pave the way for effective interventions, transforming the landscape of Alzheimer’s treatment.